Medicover Integrated Clinical Services
Your Partner inClinical Development||Companion Diagnostics
Therapeutic expertise
Turning complex diseases into breakthrough treatments
We provide comprehensive clinical services, study execution and consulting services covering a wide range of therapeutic fields, such as oncology, immunology, CNS, vaccines, rare diseases, and more.
Solutions
Clinical services to increase success rates for drug development
Experience clinical excellence with our integrated clinical solutions. We conduct whole clinical trial: from finding the right patient, applying effective drugs, managing patient samples to collecticng samples for further trials.
Central Lab
Services
Comprehensive solutions to coordinate every aspect of clinical trial laboratory services. We combine our professional experience and operational excellence to support clinical trial sponsors and CROs starting from study setup to the final submission of laboratory results.
Companion
Diagnostics
We excel in providing tailored solutions to enhance personalized patient care. Our expertise spans the development and implementation of cutting-edge diagnostic tools that complement specific therapies.
Precision
Medicine
Complete multi-omics services to support targeted therapy and drug development programs. We provide end-to-end services including biomarker discovery, target validation, CDx assay development, commercialization, and regulatory services.
Site Management
Organisation
With access to major clinical study sites and a large network of more than 100 clinical centers, we successfully integrate digitization and innovative solutions supporting patient recruitment and the whole clinical trial journey.
Laboratory services
Over 8,000 validated assays available for clinical services
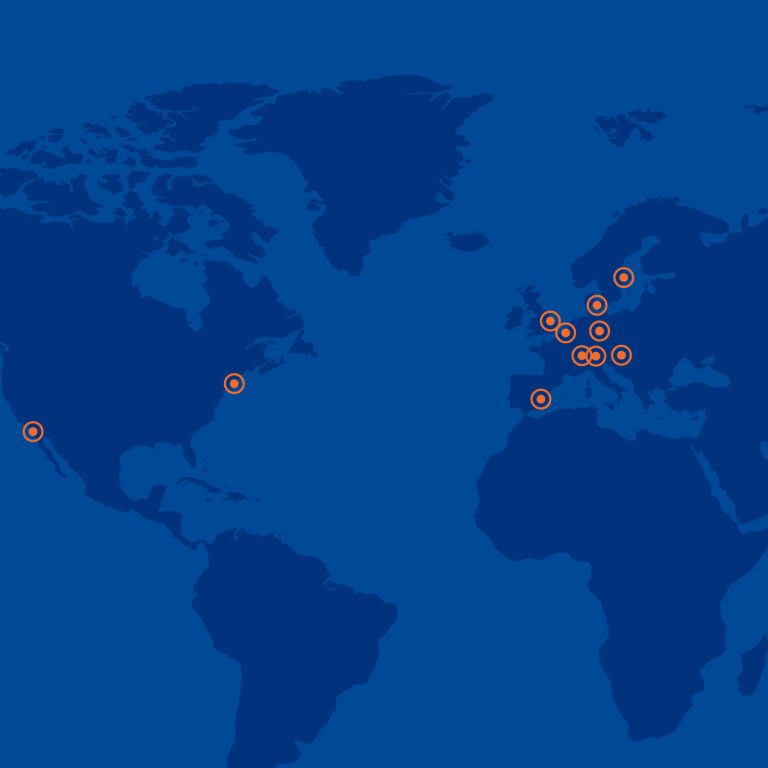
We constantly increase our analytical capabilities, conducting laboratory services in more than 100 large laboratories. You can benefit from our network of regional hubs located in 13 countires, mainly across Central and Eastern Europe, and India.
Immunoassays
Cell-Based Assays
Histology
RT-PCR
Flow Cytometry
HPLC
Coagulation
Enzymatic Assays
Platelet Function Assays
Multiplex Assays






Why us
Innovation, accuracy and global lab network
For over 20 years, we have strategically enabled Medicover’s assets, which include state-of-the-art diagnostic laboratories, medical clinics, hospitals, and the extensive expertise and enthusiasm of professionals dedicated to ensure the smooth operation of clinical trial processes.
Medicover has 100+ Medicover clinics, 100+ clinical laboratories, 2500+ global Medicover network partners and over 200 hospitals to support our patient recruitment. Our diagnostic and clinical services on-line channel reaches more than 2.1 million individuals per month, where we can find targeted patients for each clinical trial.
Quality is our guiding light in carrying out clinical trials. We continually monitor both data quality and laboratory operations to assure that the highest standards (GCP, GLP, GCLP, ISO) are maintained.
Customer references
We’re pleased to support our global partners in medical research, taking pride in assisting Pharma, Biotech, Biopharma, and CRO companies in their drug development endeavors. We appreciate mutual commitment and close cooperation that brings positive outcomes of clinical trials.
News & updates
Resourses
Browse resourses by tags:
scroll ↑↓